The Blood Service will transfer unused cord blood products to the biobank for research purposes
The Blood Service has begun a project to utilise unused cord blood products in medical research. In August, approximately one thousand mothers and their children who have donated cord blood will receive a letter from the Blood Service asking them for permission to transfer the donated cord blood to the Blood Service’s biobank.
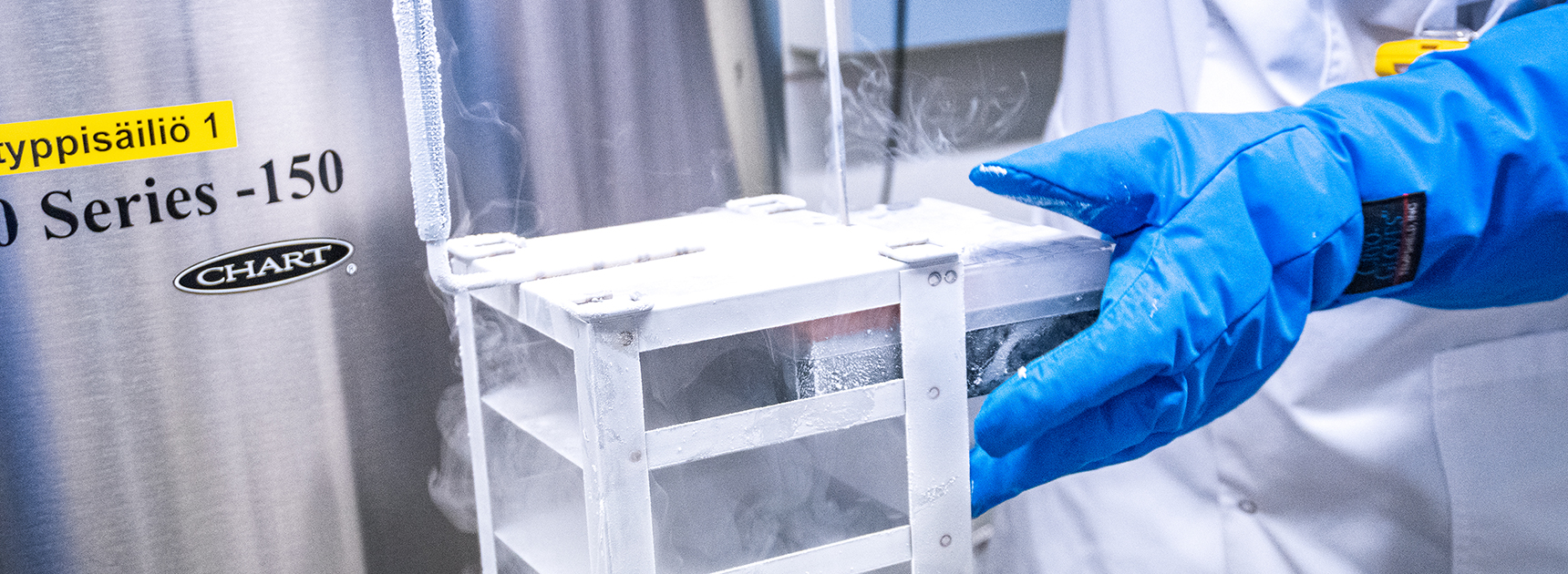
Between 1999and 2013, approximately 3,300 units of cord blood were collected for the Blood Service’s cord blood bank from healthy, volunteer mothers who had given birth. Cord blood can be used as a stem cell transplant to treat types of leukaemia and other severe blood disorders in patients who cannot find a conventional stem cell transplant.
In total, around 70 cord blood transplants from the Blood Service’s cord blood bank were used to treat patients during the 2000s. However, recent medical advances have introduced other, more effective options for treating patients. One challenge with using cord blood transplants is ensuring sufficient cell numbers for effective treatment. In recent years, there was no need for such transplants, even annually.
“Transferring products stored in the cord blood bank to the biobank means that they can be used for the benefit of patients and medicine, instead of leaving them unused in storage,” says Chief Physician Matti Korhonen from the Blood Service.
The Ethics Committee of the Hospital District of Helsinki and Uusimaa has evaluated the Blood Service’s plan for transferring cord blood to the biobank and issued a favourable statement.
Mothers and children will be asked for consent to use cord blood in the biobank
The Blood Service will send requests for consent to approximately one thousand mothers from whom cord blood was collected during birth. The same request for consent will be sent to the child whose cord blood was collected. These children are now legally adults and have the right to make the decision to transfer their cord blood for research purposes.
A mother and child who receive a transfer request may give their consent to transfer the cord blood product to the biobank or prohibit the transfer. If they give their consent, the Blood Service will transfer the donated cord blood along with blood samples taken in connection with the donation and health data to the biobank for research purposes. If they do not unanimously approve the transfer, the product will remain in storage in the Cord Blood Bank. If they wish, they can also completely prohibit the use of the cord blood in both the Cord Blood Bank and the Biobank, in which case the Blood Service will dispose of the cord blood and other materials appropriately.
The Blood Service is not able to contact the child directly. A request for the use of cord blood in the biobank is sent to the mother, who is asked to submit the request for consent to the child as well.
Cord blood is technically the child’s blood
During childbirth, the placenta and the blood it contains are unnecessary tissue, which is usually destroyed after childbirth because it is no longer required by the mother or child. The placenta contains the child’s blood, as well as the child’s genotype, i.e. DNA.
In addition to the child’s cord blood itself, a stored cord blood unit also contains a blood sample from the mother, data from a health information questionnaire filled in by the mother at the time, and a birth record containing information about the health of the mother and the child. This is why both the mother and child must consent to the transfer of the cord blood unit to the biobank.
Biobanks promote medicine and health care
Biobank research supports the development of health care. Biobank samples and data are used to investigate the causes of diseases and the impact of genotype, environment and lifestyle on their origin. They can also be used to develop new types of treatment. Biobank data can also be utilised in product development for medicines. Like the Blood Service’s other activities, biobank operations are also non-profit.
There are several different biobanks operating in Finland, some in connection with hospital districts. The Blood Service’s Biobank was established in 2017, and so far it has collected blood donor samples and data.
All material is processed and stored with strict respect for privacy. The samples and related information are coded when they are given to researchers so that they cannot be used to identify the person. Biobank operations are regulated, among other things, by the Biobank Act and are supervised by the Finnish Medicines Agency Fimea. Biobanks only provide samples and data for research that meets the required scientific and ethical criteria.