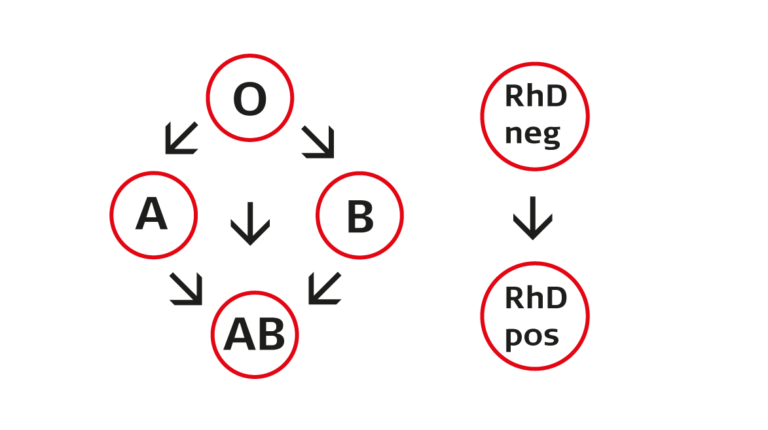
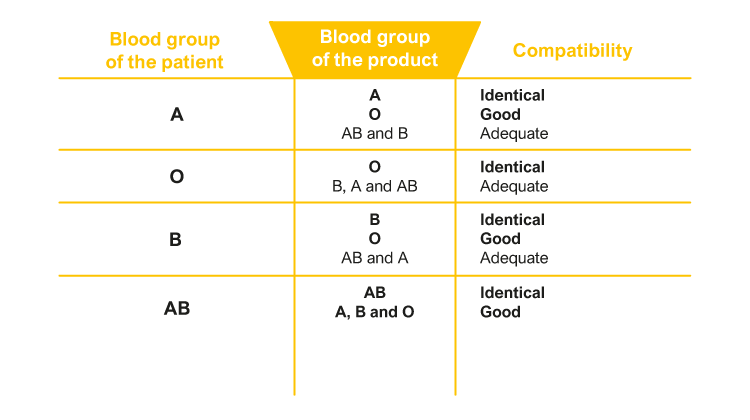
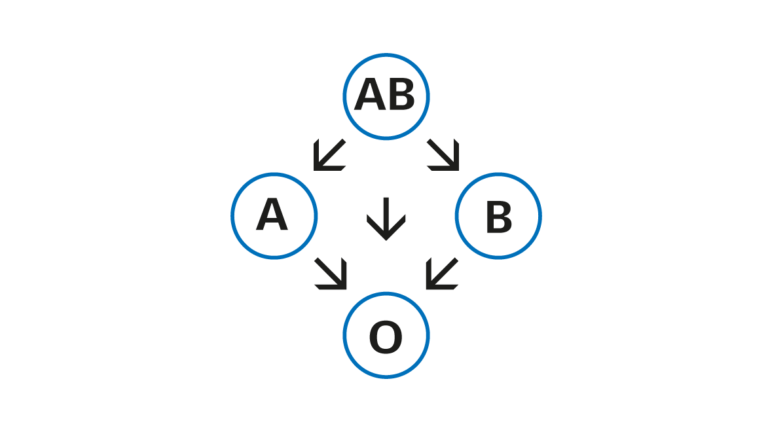
Instructions for blood transfusions
Blood being a biological material, its use requires great care and good orientation. Correct identification of the patient, selection of the right product, and compatibility testing of blood products well in advance are the key factors in ensuring the safety of blood transfusions.
Preparing for a blood transfusion
- It is important to prepare for a blood transfusions, especially if the patient has a rare blood group, multiple red cell antibodies, IgA deficiency, HLA or HPA immunisation or other need for a special blood product.
- The hospital’s blood bank answers for the pretransfusion blood group testing. The Blood Service helps with the testing and choosing the right product if needed.
- More information about the blood products and their use can be found in the Finnish guidebook: Verivalmisteiden käytön opas.
Things to consider in the nursing unit before blood transfusion
- A red blood cell transfusion must be completed within 6 hours after bringing the product to room temperature.
- Platelet products must be stored and transfused at room temperature.
- The patient’s illnesses and clinical condition must be considered in the infusionrate.
- The need for pre-medication should be concsidered (e.g. antipyretic, antihistamine, diuretic).
Only handle blood products that are intended for one patient at a time!
- Make sure that the product matches the patient (blood group, compatibility test, special products such as irradiated products).
- Inspect the blood product (expiry date, colour, appearance, presence of blood clots or gas).
- All blood products are transfused using transfusion equipment fitted with a filter (150–200 μm).
- Only electrolyte solutions that do not contain calcium (such as NaCl 0.9%) may be infused via the same catheter, transfusion equipment or tubing used for administering blood products.
At the bedside
- Identify the patient and make sure that the product is meant for the patient in question.
- Start every blood transfusion with the biological pre-testing, that is slowly and monitor the patient closely, because an adverse reactions may be related to the properties of an individual blood product.
- If the patient gets an adverse reaction, stop the blood transfusion and notify the doctor.
Documentation and tracability
- The blood transfusion must be documented in detail: vital signs before and after the transfusion, the start and end time of the transfusion, the amount transferred and any possible changes in the patient’s condition.
- Store the bag and the transfusion equipment for 24 h in the fridge (close them with a stopper) and the tubing and its attachments used for compatibility testing for 3 days (marked with the product unit number and personal identity sticker).
- Return any unused blood products to the hospital’s blood centre to ensure tracability.
Last modified: 15.12.2023